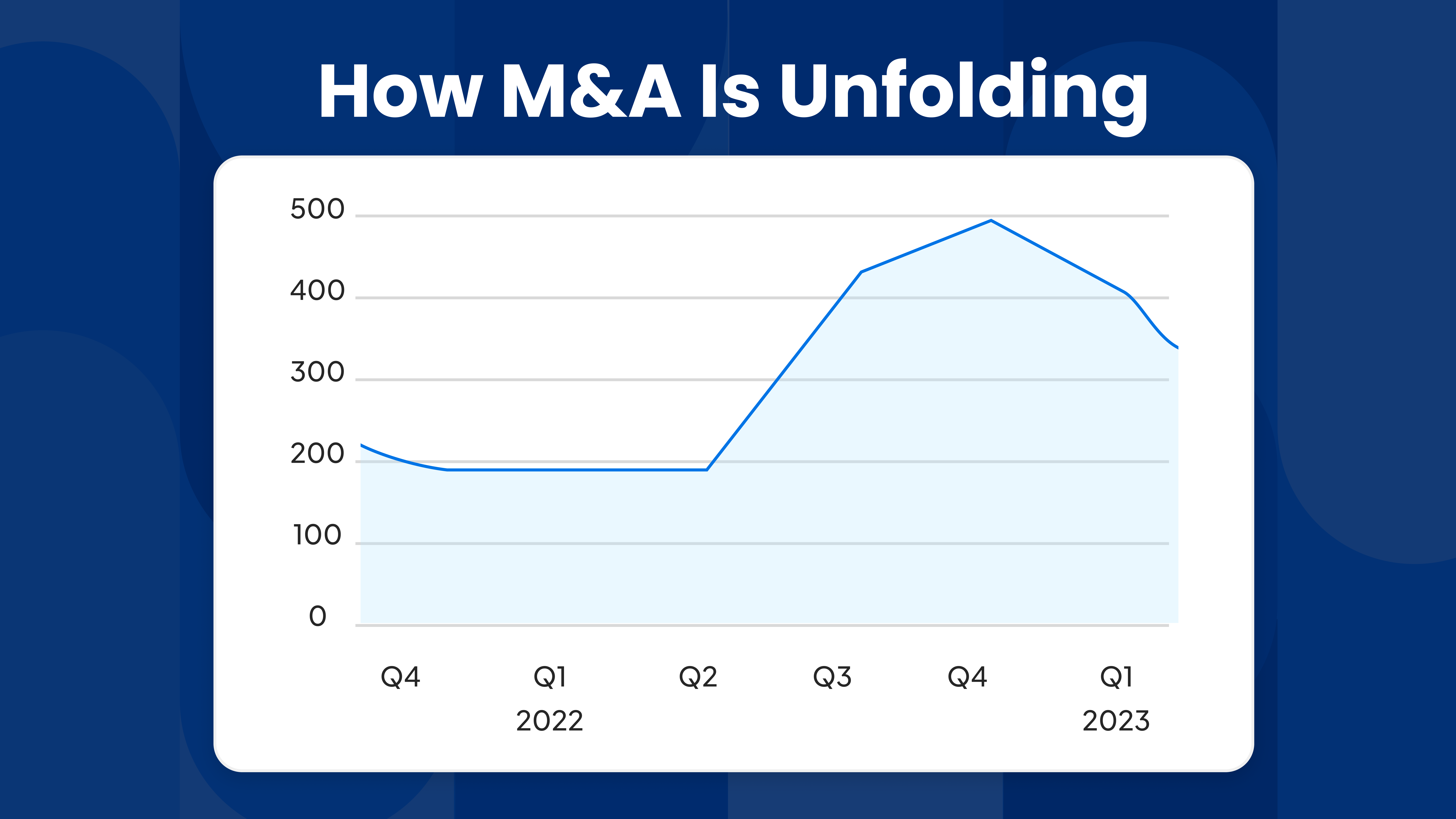
With obesity a growing concern worldwide, clinicians and health officials are scratching their heads as to what appropriate and sustainable lines of treatment there are. Obesity, along with other non-communicable diseases like diabetes and hypertension, has major implications for healthcare systems.
Apart from expensive and hazardous surgery, the other option is weight-loss drugs. These have understandably hit the market with huge aplomb. Losing weight through the so-called “traditional” means of diet and exercise has failed miserably for millions of people.
Weight loss drugs Wegovy and Ozempic are self-administered weekly injectables. They mimic a human hormone called GLP-1. This hormone is released in the gut in response to eating and signals fullness much quicker. Generally, semaglutide has been used in treating Type 2 diabetes, but is effective in addressing obesity.
The drugs are quite complex to use, and come with a hefty price tag. Indeed, the lowest price for a month’s supply of semaglutide at the maximum dose for weight loss is $804 in the US.
This is why the field is ripe for new and improved ways to treat obesity
Introducing startups like Fracty, Health. This biotech company aims to simplify treatment, reduce cost, and ensure the longevity of results. Indeed, with weight loss drugs like Ozempic, once someone stops taking the ‘dose’, the weight piles back, and usually with interest.
Fractyl Health has developed a treatment for Type 2 diabetes with uses for weight control. It is hoped that this could be a ‘one-and-done’ treatment that lasts for years. Essentially, the company aims to use gene therapy to deliver an artificial gene to the pancreas to continuously produce the GLP-1 hormone.
Gene therapy is a fascinating and exciting field for biotech companies and lifescience entrepreneurs. In this instance, the treatment usesinactivated viruses to carry a therapeutic gene to pancreatic cells. Viruses are used because of their natural ability to deliver genetic material to cells.
What opportunities exist for biotech in sustainable weightloss interventions?
The bottom line is that innovative treatment is needed to tackle obesity. Many things cause this disease; biotech can consider numerous ways to contribute to treatment. Broadly, biotech can leverage advancements in genetics, microbiome research, pharmaceuticals and personalized medicine. Possibilities include:
- Precision medicine and genetic testing
Biotech can use genetic testing to identify individuals’ genetic predispositions to weight gain and obesity. There’s an opportunity to develop personalized weight loss plans and interventions based on genetic profiles to optimize outcomes.
- Microbiome modulation
Research and development interventions that target the gut microbiome to influence metabolism and weight regulation look promising. This could involve probiotics, prebiotics or microbiome-based therapies.
- Pharmaceutical innovations
Drug discovery that targets specific biological pathways involved in appetite regulation, fat metabolism and energy expenditure should be invested in. These drugs, combined with lifestyle changes to enhance weight loss efforts, may be effective in the long term.
- Nutrigenomics
Biotech companies can study the interaction between nutrients and genes to tailor dietary recommendations based on a person’s genetic makeup. Personalized nutrition plans could be designed.
- Neuroscience and brain-machine interfaces
Lifescience innovators could investigate how the brain processes hunger and satiety signals. Interventions could, for example, use brain-machine interfaces or neurostimulation to regulate appetite and promote healthier eating behaviors.
- Cellular and gene therapies
Cellular therapies, which include adipose-derived stem cells, could target fat tissue and promote healthy weight loss. Gene therapies that modify genes related to metabolism and fat storage present significant opportunities for biotech entrepreneurs.
- Bariatric surgery innovations
There is great potential for scientists to develop minimally invasive and safer bariatric surgery techniques. These could have fewer complications and be less expensive, making weight loss surgery a more accessible and sustainable option.
- Metabolic engineering
It is possible to engineer metabolic pathways to enhance energy expenditure and reduce fat storage. Biotech entrepreneurs could develop therapies that target specific metabolic processes to promote weight loss.
- Combination therapies
Investigating the synergistic effects of combining different biotechnological approaches, such as drugs, genetic interventions and behavioral strategies to create sustainable weight loss interventions is a critical gap.
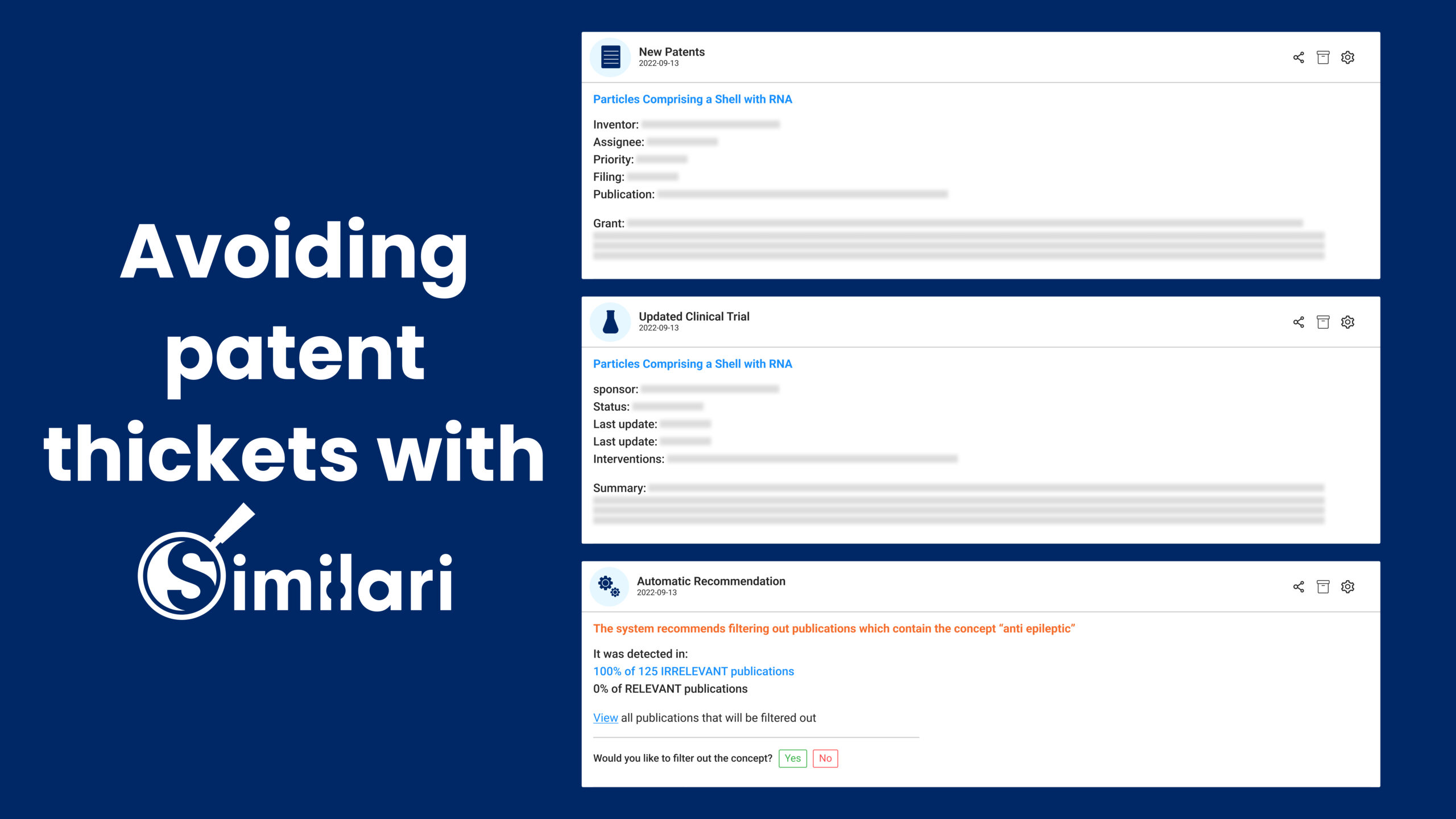
No need to tighten your belts with Similari
There are a myriad of weight loss innovations with potential. They also come with ethical, safety and regulatory considerations. Rigorous research, clinical trials and collaboration between researchers and healthcare professionals are essential.
With Similari’s AI-enabled intelligence platform, you can gauge the weightloss drug discovery and innovation landscape in granular detail. You’ll be able to forge ahead knowing what your competitors are up to, who has secured patents and who has concluded a trial. Similari trains itself over time and delivers top insights relevant to your industry.
Book a demo today to find out how you can offload 90% of your research time with Similari.